Translation services for Clinical Study Reports (CSRs) in the UK are essential for companies submitting to regulatory bodies like the Medicines and Healthcare products Regulatory Agency (MHRA). These specialized translation services must ensure scientific and linguistic accuracy, particularly when dealing with complex medical terminology. The process involves using a combination of expert multilingual translators, subject matter experts (SMEs), advanced translation technology, and thorough quality assurance measures to guarantee that the translated CSRs accurately reflect the original study data and comply with UK regulatory standards. This meticulous approach not only streamlines the review process but also ensures that international research findings are effectively communicated and understood by UK regulators, thereby facilitating the advancement of clinical trials on an international scale. The end goal is to submit CSRs that have been accurately translated into English are crucial for maintaining the validity of the study's progress and potentially delay the approval and distribution of medical treatments and therapies in the UK market but also across different regions where English is the target language, ensuring compliance with both local and international standards. This results in a transparent, reliable translation process that meets regulatory requirements and supports the global dissemination of clinical research, ultimately enabling researchers to navigate the complexities of international regulation effectively.
Navigating the complex landscape of pharmaceutical regulation, companies are often required to submit Clinical Study Reports (CSRs) for UK approval. This process is pivotal in ensuring patient safety and treatment efficacy. As clinical trials increasingly involve multinational collaboration, the necessity for accurate translation services for CSRs UK becomes paramount. This article delves into the critical aspects of translating CSRs to meet UK regulatory standards, from comprehending the importance of these reports to identifying reliable translation service providers. We explore the intricacies of UK regulatory requirements, best practices in translation methodologies, and the challenges faced during this process. With a focus on maintaining scientific rigor and data integrity, we provide a comprehensive guide that encompasses legal and ethical considerations, technological advancements, and post-translation quality assurance to ensure CSRs align with UK standards. This article serves as an invaluable resource for pharmaceutical companies aiming to submit translated CSRs to the UK, ensuring their research is accurately conveyed and embraced by UK regulatory bodies.
- Understanding the Importance of Clinical Study Reports (CSRs) for UK Submission
- Overview of Regulatory Requirements for CSRs in the UK
- The Role of Translation Services in CSRs for UK Authorities
- Key Considerations When Translating Clinical Study Reports into English
- Identifying a Reliable Translation Service Provider for CSRs
- The Process of Translating CSRs: Best Practices and Methodologies
- Ensuring Accuracy and Compliance in Translated CSRs for UK Submission
- Challenges and Solutions in Translating Clinical Study Reports
- Case Studies: Successful CSR Translations and Submissions to UK Regulatory Bodies
- Checklist for Finalizing Translated Clinical Study Reports Ready for UK Submission
Understanding the Importance of Clinical Study Reports (CSRs) for UK Submission
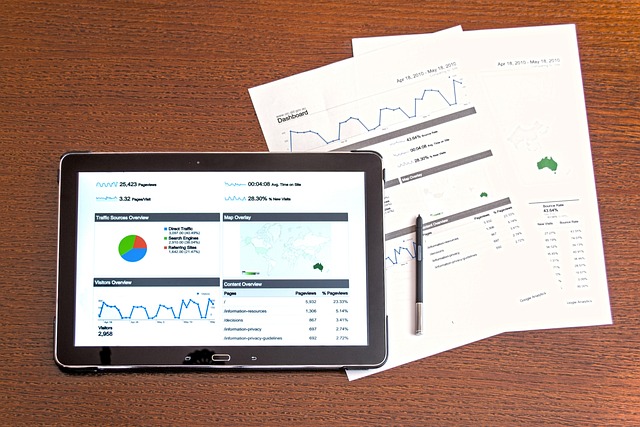
When preparing for a UK submission, the clinical study reports (CSRs) are pivotal documents that offer regulators a comprehensive overview of the clinical development program, including trial design, methodology, results, and conclusions. These reports are critical as they serve as the primary source of information on which regulatory decisions regarding marketing authorization or product approval are based. In this context, translation services for CSRs play an indispensable role, particularly when the original documents are in a language other than English. Engaging professional translation services for Clinical Study Reports (CSRs) UK ensures that these vital documents meet the stringent requirements of the UK regulatory bodies, such as the Medicines and Healthcare products Regulatory Agency (MHRA). This is crucial for maintaining the integrity of clinical data and facilitating a smooth review process. The translated CSRs must be accurate and precise to reflect the original content’s nuances, ensuring that no critical information is lost or misinterpreted. Utilizing specialized translation services for Clinical Study Reports (CSRs) UK submission not only demonstrates compliance with regulatory standards but also enhances the efficiency of the review process, ultimately expediting the time frame for product availability in the UK market.
Overview of Regulatory Requirements for CSRs in the UK
In the United Kingdom, regulatory requirements for Clinical Study Reports (CSRs) are stringent and meticulously defined to ensure the integrity and quality of clinical research. Sponsors conducting clinical trials must adhere to the guidelines set forth by the Medicines and Healthcare products Regulatory Agency (MHRA), which is responsible for ensuring that medicines and medical devices work and are safe. A pivotal aspect of this compliance involves the translation of CSRs, as these documents must be submitted in English when applying for marketing authorization in the UK. Translation services specialized in CSRs for the UK market are essential to navigate these requirements effectively. These services ensure that all critical data and nuances within the original reports are accurately captured and conveyed in the target language, thereby facilitating a smooth review process by the regulatory authorities. The translations must not only be linguistically precise but also reflect the scientific context accurately, as any discrepancies could lead to delays or complications in the approval process.
The translation of CSRs into English for UK submission is not merely a matter of language conversion; it entails a comprehensive understanding of both the regulatory environment and the clinical trial context. Translation services for CSRs must be carried out by professionals with expertise in both the life sciences domain and the regulatory requirements specific to the UK. This ensures that the translated reports meet the high standards expected by the MHRA, as well as maintain the original study’s context, intent, and integrity. The involvement of experienced translators who are adept at handling complex scientific terminology and familiar with the submission process in the UK is crucial for the successful navigation of these regulatory hurdles.
The Role of Translation Services in CSRs for UK Authorities

When clinical study reports (CSRs) are prepared for regulatory submission in the UK, translation services play a pivotal role to ensure that the content is comprehensible and compliant with local regulations. The UK’s stringent guidelines mandate that all CSRs submitted for marketing authorization must be accurately translated into English if they were initially drafted in another language. This is not merely a matter of linguistic accuracy but also a question of regulatory compliance, as the Medicines and Healthcare products Regulatory Agency (MHRA) requires that translations maintain the integrity and meaning of the original text.
Translation services for CSRs UK must be adept in specialized terminology, adhering to the Good Clinical Practice (GCP) and the International Conference on Harmonisation (ICH) guidelines. These services ensure that the translated reports accurately reflect the clinical findings, methodologies, and results as intended by the original authors. The reliability of these translations is critical, as they form the basis for UK authorities to assess the safety and efficacy of medicinal products. Thus, employing professional translation services with expertise in the pharmaceutical sector is essential for a successful regulatory submission process in the UK.
Key Considerations When Translating Clinical Study Reports into English

When translating clinical study reports (CSRs) into English for submission in the UK, precision and expertise are paramount. The translation services for CSRs must adhere to stringent regulatory standards, as the accuracy of translated content can significantly influence clinical trial outcomes and subsequent regulatory decisions. Key considerations include selecting translators with a deep understanding of both the source language and the clinical research terminology. These professionals should have relevant qualifications and experience in the field of translation for CSRs, ensuring their ability to convey complex scientific information accurately without loss of meaning or nuance. It is essential that the chosen translation service has a proven track record of working with regulatory agencies, such as the Medicines and Healthcare products Regulatory Agency (MHRA), to ensure that the translated reports meet all legal and linguistic requirements for submission in the UK. The translation process should involve careful validation of terms and concepts, particularly those unique to clinical research, to guarantee that the integrity of the original report is upheld in its English equivalent. This meticulous approach not only facilitates efficient regulatory review but also supports the credibility and validity of the clinical study outcomes within the UK landscape.
Identifying a Reliable Translation Service Provider for CSRs

When navigating the intricate process of submitting Clinical Study Reports (CSRs) to regulatory authorities in the UK, the importance of precise and accurate translations cannot be overstated. The translation of CSRs from their original language into English is a critical step that demands expertise not only in linguistics but also in the specialized field of clinical research. Identifying a reliable translation service provider for CSRs UK entails a thorough vetting process to ensure compliance with regulatory standards such as the Medicines and Healthcare products Regulatory Agency (MHRA) guidelines. Translation services for Clinical Study Reports UK must be proficient in handling complex medical terminology, statistical data, and sensitive information with confidentiality and professionalism. It is imperative to choose a provider that boasts a track record of successful translations in the clinical research domain, one that has demonstrated a deep understanding of both the source and target languages, as well as the scientific context. This ensures that the translated CSRs retain their original meaning and are ready for submission without compromising on quality or integrity. A provider with expertise in this niche not only accelerates the submission process but also enhances the likelihood of a successful review by regulatory bodies. In doing so, they act as a bridge between research entities and the UK’s stringent regulatory framework, facilitating smoother and more efficient clinical trial processes.
The Process of Translating CSRs: Best Practices and Methodologies

When navigating the translation of Clinical Study Reports (CSRs) for submission in the UK, utilising specialised translation services is paramount. These reports are critical documents that provide a comprehensive account of clinical trial methodology and patient outcomes, ensuring that they meet stringent regulatory standards. The process of translating CSRs involves not just linguistic accuracy but also scientific precision to convey the nuances of clinical trials accurately.
The best practices in translating CSRs begin with selecting a translation service that specialises in both the source and target languages, particularly those well-versed in medical terminology. This expertise is crucial for maintaining the integrity of the data and ensuring that the translated report adheres to the regulatory requirements set forth by the Medicines and Healthcare products Regulatory Agency (MHRA) or other relevant UK bodies. Employing native linguists with a background in the clinical sciences guarantees a thorough understanding of both the language and the subject matter. Furthermore, using translation memory software and terminology databases tailored to medical fields can streamline the process, ensuring consistency across all translated CSRs and facilitating faster turnaround times without compromising on quality. This combination of human expertise and technological efficiency is key to successful translations that withstand scrutiny by regulatory authorities and contribute to the smooth progression of clinical trials in the UK landscape.
Ensuring Accuracy and Compliance in Translated CSRs for UK Submission

When navigating the complex process of submitting Clinical Study Reports (CSRs) to regulatory authorities in the UK, precision and compliance are paramount. Translation services for CSRs destined for UK submission must not only accurately convey the scientific content but also align with the stringent guidelines set forth by agencies such as the Medicines and Healthcare products Regulatory Agency (MHRA). To ensure the integrity of the data, it is essential to engage translation professionals who are not only linguistically proficient but also well-versed in the technicalities of clinical research terminology. These experts must be adept at translating from the original language to English while maintaining the report’s scientific validity and regulatory compliance. The translated CSRs should reflect the same level of detail, clarity, and data integrity as the source documents, enabling UK regulators to assess the study’s findings accurately and efficiently. Utilizing specialized translation services for CSRs tailored for UK submission can mitigate the risks associated with mistranslation or misinterpretation, thereby facilitating a smoother review process and maintaining the study’s credibility in an international context.
Challenges and Solutions in Translating Clinical Study Reports

The translation of Clinical Study Reports (CSRs) from their original language to English for UK submission presents a complex array of challenges that must be carefully navigated. One primary concern is maintaining the integrity and accuracy of scientific data across different languages, as mistranslations can lead to critical misunderstandings and potential setbacks in the regulatory process. The intricacies of medical terminology and the specific context within CSRs necessitate translation services with expert linguistic proficiency and a deep understanding of clinical research vernacular. Language service providers (LSPs) specializing in CSR translations must employ subject matter experts (SMEs) who are not only fluent but also knowledgeable about clinical trial methodologies and regulatory requirements, such as the guidelines set forth by the European Medicines Agency (EMA) or the U.S. Food and Drug Administration (FDA).
To overcome these challenges, collaboration between multilingual translators and experienced SMEs is essential. Advanced translation technologies, such as translation memory systems and machine learning algorithms, can further enhance the precision and consistency of the translated content. These tools aid in recalling previously translated segments and maintaining a uniform terminology throughout the document. Furthermore, quality assurance processes involving peer reviews by experts in both the source and target languages ensure that the final CSR reflects the original content’s scientific rigor and regulatory compliance. By implementing such solutions, translation services for Clinical Study Reports can effectively bridge the language gap, allowing researchers to present their findings to regulatory bodies like the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK with confidence.
Case Studies: Successful CSR Translations and Submissions to UK Regulatory Bodies
Pharmaceutical companies conducting clinical trials in the UK must ensure that their Clinical Study Reports (CSRs) are accurately translated to meet the regulatory requirements set forth by UK bodies such as the Medicines and Healthcare products Regulatory Agency (MHRA). A prime example of successful CSR translation involves a global biotech firm that required precise translations of its CSR from Japanese to English for a pivotal phase III trial. Utilizing specialized translation services for Clinical Study Reports UK, the company’s report was meticulously translated, capturing the nuances and scientific precision necessary for regulatory submission. This translation facilitated a seamless review process, resulting in the trial progressing without delay. Another case highlighted the importance of cultural competence in translations. A multinational pharmaceutical company faced challenges when translating CSRs from English to Spanish for a Latin American market. The chosen translation services for Clinical Study Reports UK not only provided linguistic accuracy but also adapted the content to align with local regulatory standards and patient-centric considerations, ensuring the study’s findings were appropriately understood and evaluated by local regulators. These case studies underscore the critical role of expert translation services in the successful submission of CSRs to UK regulatory bodies, enabling companies to navigate the complexities of international clinical trials with confidence.
Checklist for Finalizing Translated Clinical Study Reports Ready for UK Submission

When finalizing translated clinical study reports (CSRs) for UK submission, adherence to a comprehensive checklist is paramount. This ensures that all translations meet the stringent regulatory standards required by the Medicines and Healthcare products Regulatory Agency (MHRA). The checklist should begin with verifying the credentialsof translation services for CSRs UK to ascertain their expertise in handling scientific documents within the healthcare sector. Each translation must be performed by a subject-matter expert who is proficient not only in the source and target languages but also well-versed in clinical study report terminology.
Upon completion of the translation, a thorough review process is essential. This includes checking for accuracy in the translation of medical terms, coherence of the narrative, and alignment with the original document’s content and intent. Any discrepancies or omissions must be rectified promptly. Additionally, ensuring that the translations comply with the Good Clinical Practice (GCP) guidelines is crucial. The final step is to confirm that all required documents, including informed consent forms if applicable, are accurately translated and attached. By meticulously following this checklist, sponsors can ensure their translated CSRs for UK submission are ready for regulatory review and meet the high standards expected by the MHRA.
In concluding, the meticulous preparation and translation of Clinical Study Reports (CSRs) for submission in the UK is a multifaceted process that demands precision, regulatory adherence, and linguistic expertise. Utilizing specialized translation services for CSRs UK-bound is not merely a procedural step but an integral component of successful pharmaceutical evaluations. By adhering to the outlined best practices, from selecting a dependable service provider to ensuring the translated reports meet both linguistic and regulatory standards, sponsors can navigate this critical phase with greater assurance. The case studies presented underscore the effectiveness of well-translated CSRs in securing approval from UK regulatory bodies. With the provided checklist as a guide, pharmaceutical companies can confidently submit their translated CSRs, paving the way for advancements in healthcare and patient welfare.
