Clinical Study Reports (CSRs), vital documents in drug development, require meticulous translation services to ensure compliance across regions under UK regulations via the MHRA. Professional translators must master clinical terminology and cultural nuances to avoid misinterpretations and streamline global trial submissions. Specialized CSR translation services use advanced technology, quality assurance processes, and terminological databases to meet stringent UK standards, facilitating accurate regulatory submissions. AI and Machine Translation (MT) are transforming this field, offering faster, cost-effective solutions while maintaining accuracy and compliance for the growing demand of multinational clinical trials.
Are your clinical study reports (CSRs) compliant with UK regulations? Navigating the complex landscape of healthcare documentation can be a challenge, especially when global collaborations demand accurate and regulated translations. This comprehensive guide explores the intricacies of UK CSR regulations, emphasizing the pivotal role of language and translation services in ensuring adherence. We delve into common challenges, best practices, quality assurance measures, and future trends shaping the landscape of translation services for CSRs in the UK.
- Understanding UK Regulations for Clinical Study Reports (CSRs)
- The Role of Language and Translation in CSR Compliance
- Common Challenges in Ensuring CSR Accuracy and Adherence
- Best Practices for Accurate and Compliant CSR Translation
- Choosing the Right Translation Service Provider for CSRs
- Quality Assurance and Control Measures for CSR Translations
- Case Studies: Successful CSR Translation and Compliance
- Future Trends and Technologies in CSR Translation Services
Understanding UK Regulations for Clinical Study Reports (CSRs)

Clinical Study Reports (CSRs) are a critical component in the drug development process, providing detailed accounts of clinical trials. Ensuring compliance with UK regulations is paramount for pharmaceutical companies aiming to bring products to market seamlessly. The landscape of CSR regulation in the UK is intricate, involving guidelines set forth by the Medicines and Healthcare products Regulatory Agency (MHRA). These guidelines encompass data integrity, reporting standards, and quality requirements, all designed to safeguard patient safety and ensure the reliability of clinical trial data.
Translation services play a pivotal role in navigating these regulations, especially for multinational pharmaceutical firms conducting trials across diverse regions. Accurate and linguistically adept CSR translation ensures that regulatory bodies worldwide, including those in the UK, receive consistent and comprehensible documentation. This process involves not just word-for-word translation but also understanding the nuances of clinical terminology to maintain data integrity and accuracy across languages.
The Role of Language and Translation in CSR Compliance

Clinical Study Reports (CSRs) require precise and clear language to ensure compliance with UK regulations. Accuracy in CSR content is paramount, as any ambiguity or inconsistency can hinder regulatory approval or lead to legal issues. Therefore, professional translation services are often essential for multinational clinical trials, ensuring that CSR documentation accurately reflects the study’s intricacies across different languages.
When it comes to translation, choosing qualified translators specializing in medical terminology and clinical research is crucial. Translation services for CSRs in the UK must consider cultural nuances as well, to avoid misinterpretations or inappropriate localizations. This meticulous approach guarantees that regulatory bodies can easily verify the report’s integrity and adherence to guidelines, streamlining the approval process.
Common Challenges in Ensuring CSR Accuracy and Adherence

Ensuring accuracy and compliance in Clinical Study Reports (CSRs) is a complex task, particularly when navigating the stringent regulations of the UK. One of the primary challenges lies in maintaining consistency and precision throughout the document translation process. CSRs often require multiple languages for international clinical trials, introducing potential errors during interpretation and localization. Professional translation services play a vital role in mitigating this risk, offering specialized medical terminology and cultural expertise to bridge language gaps.
Another hurdle is adhering to specific formatting and presentation standards set by regulatory bodies. UK regulations demand detailed, structured reports with consistent terminology and data presentation. Non-compliance may lead to significant delays and rejections during the review process. Reputable translation companies specializing in CSRs can assist in formatting and ensuring that translated documents meet these exacting standards, thereby streamlining the overall submission process for clinical trials conducted across diverse global markets.
Best Practices for Accurate and Compliant CSR Translation

When translating Clinical Study Reports (CSRs) for compliance with UK regulations, best practices involve a meticulous approach to ensure accuracy and adherence. Start by engaging professional translation services specialised in CSRs, who understand not just language but also clinical terminology. These experts should have access to up-to-date regulatory guidelines and be adept at navigating the complex requirements of the Medicines and Healthcare products Regulatory Agency (MHRA).
A rigorous quality assurance process is crucial. This includes proofreading by subject matter experts, ensuring consistency in terminology, and careful handling of technical jargon to preserve the integrity of the scientific content. Translation memory tools can help maintain consistency across documents, while real-time collaboration platforms facilitate communication between translators, reviewers, and clients, ensuring every detail aligns with UK regulations for Clinical Study Reports.
Choosing the Right Translation Service Provider for CSRs

When it comes to ensuring your Clinical Study Reports (CSRs) are compliant with UK regulations, selecting the right translation service provider is a crucial step in the process. It’s not just about finding someone who can translate words from one language to another; you need specialists who understand the unique terminology and requirements of pharmaceutical documentation. Look for providers with extensive experience in translating CSRs, who employ native speakers, and who are familiar with Good Clinical Practice (GCP) guidelines.
Choosing a provider that offers quality assurance processes such as proofreading, editing, and review by subject matter experts ensures accuracy and consistency. Additionally, consider their technological capabilities; many reputable translation services now offer specialized tools for managing large volumes of data and ensuring terminological consistency across multi-language CSRs. This not only streamlines the translation process but also helps maintain regulatory compliance.
Quality Assurance and Control Measures for CSR Translations

Ensuring accurate and compliant CSR translations is paramount in the UK, where stringent regulations govern clinical study reports. Quality Assurance (QA) and Control Measures are essential to maintain data integrity and precision during translation processes for CSRs. Reputable translation services for Clinical Study Reports (CSRs) UK follow rigorous QA protocols, including thorough language proficiency testing of translators and editors. Each stage of the translation workflow is meticulously reviewed, from initial evaluation to final proofreading.
Control measures involve implementing standardized procedures, such as memory management systems and terminological databases, to guarantee consistent terminology usage. Advanced software tools aid in identifying potential errors and ensuring formatting compatibility with original CSRs. These comprehensive QA and control practices are vital to deliver high-quality, reliable translations, thereby facilitating accurate regulatory submissions for clinical trials in the UK.
Case Studies: Successful CSR Translation and Compliance

Clinical Study Reports (CSRs) are a critical component in the drug development process, and ensuring their compliance with UK regulations is paramount. Translation services play a vital role here, especially for multinational pharmaceutical companies conducting trials across diverse regions. Successful CSR translation involves more than just converting text from one language to another; it demands an understanding of the source document’s technical terminology and regulatory requirements.
Case studies highlight the impact of professional translation services in navigating complex UK regulations. For instance, a global pharma company conducting a clinical trial in rural areas across Europe faced challenges translating CSRs into multiple languages while adhering to local guidelines. By engaging specialized translation experts, they achieved seamless communication, ensuring their study reports met all necessary standards. This success story underscores the importance of translation services for CSRs, enabling companies to maintain compliance and streamline their global clinical research endeavors.
Future Trends and Technologies in CSR Translation Services
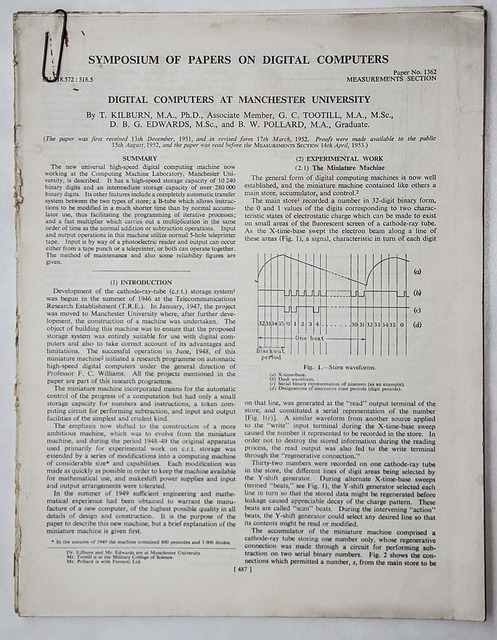
The future of translation services for Clinical Study Reports (CSRs) in the UK looks set to be shaped by several emerging trends and technologies. Artificial Intelligence (AI) and Machine Translation (MT) are becoming increasingly sophisticated, with advanced neural machine translation models capable of delivering high-quality translations. These tools can significantly speed up the CSR translation process, making it more efficient and cost-effective.
Moreover, as global clinical trials become the norm, there’s a growing demand for specialized translation services that cater to technical and regulatory requirements. Advanced post-editing tools and human review processes will remain integral to ensuring accuracy and compliance with UK regulations. The integration of these technologies promises a seamless transition towards more streamlined and precise CSR translation services, meeting the evolving needs of the pharmaceutical industry in the digital age.
Ensuring your Clinical Study Reports (CSRs) comply with UK regulations is vital for accurate data dissemination and ethical research practices. By understanding these regulations, leveraging language and translation expertise, and adopting best practices, you can navigate the complexities of CSR preparation. Choosing reputable translation service providers who employ robust quality assurance measures further enhances compliance. With a focus on technology and continuous improvement, translation services for CSRs in the UK are evolving to meet the demands of modern clinical research, fostering trust and transparency in the industry.
